Velo™ Multi-Drugs Rapid Test
- VE242007(1Prep)
- VE242002(20Preps)
- VE242008(100Preps)
Kit Content
| DOA Test | Calibrator | Cut-off (ng/ml) |
| Amphetamine (AMP) | D-Amphetamine | 1000 |
| Barbiturates (BAR) | Secobarbital | 300 |
| Benzodiazepines (BZO) | Oxazepam | 300 |
| Buprenorphine (BUP) | Buprenorphine | 10 |
| Cocaine (COC) | Benzoylecgonine | 300 |
| Ketamine (KET) | Ketamine | 1000 |
| Marijuana (THC) | 11-nor-Δ9-THC-9 COOH | 50 |
| Methylenedioxymethamphetamine (MDMA) | 3,4-Methylenedioxymethamphetamine | 500 |
| Methadone (MTD) | Methadone | 300 |
| Methamphetamine (MET) | D-Methamphetamine | 1000 |
| Opiates (OPI) | Morphine | 300 |
| Phencyclidine (PCP) | Phencyclidine | 25 |
| Tramadol (TRA) | Tramadol | 200 |
| Tricyclic Antidepressants (TCA) | Nortriptyline | 1000 |
Principle
The VeloTM Multi-Drugs Rapid Test is a device composed of 12-14 chromatographic strips designed to detect 12-14 (as per the device format) individual drugs of abuse. Each strip consists of a sample pad containing antibody-dye colloidal gold conjugate and membrane contains immobilized drug conjugate and control reagent. Urine specimen initially reacts with the antibody-dye colloidal gold conjugate, and then flows onto the strip and migrates through the pads and membrane of the strip by capillary action, to the test area. If sufficient drug is present in urine, it binds with the conjugate, preventing it from binding to the drug conjugate immobilized on the membrane in the test line region (T). Any unbound conjugate continues to migrate through the strip to the control line region (C) where it binds to the control reagent and generates a reddish-purple line in the control line region (C). Presence of both a reddish-purple quality control line (C) and a reddish purple test line (T) on the strip indicate a negative test result, or the drug concentration is below limit of detection. Presence of only a reddish-purple control line (C) on the strip indicates that specific drug has been detected and test result is positive.
Sample Collection and Preparation
- Specimen Collection
Collect the urine specimen in the provided urine cup, or in a clean, dry glass or plastic container of similar size. Urine specimens collected at any time of the day may be used. Urine specimens exhibiting visible precipitates should be centrifuged, filtered, or allowed to settle to obtain a clear specimen for testing.
- Specimen Storage
Urine specimens maybe stored at 2-8°C for up to 48 hours prior to testing. For prolonged storage, specimens may be frozen and stored below -20°C. Frozen specimens should be thawed and mixed before testing.
- Preparation
Open the package and equilibrate the test and specimens to room temperature. The most suitable temperature condition to perform the testis room temperature (15~30℃). If the test kit is stored at room temperature (15~30℃), it can be opened and used immediately.
Procedures
For direct Test Strip testing
- Take out the test strip from the closed canister or sealed pouch. Use the test strip as soon as NOTE: For canister packaging, immediately close the canister tightly after taking out the required number of the test strip(s). Record the initial opening date on the canister. Once the canister has been opened, the remaining test strip(s) are stable for 90 days only.
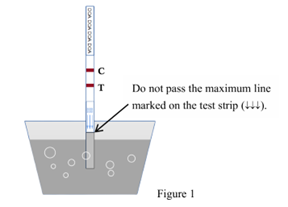
- With arrows pointing toward the urine specimen, immerse the test strip vertically in the specimen for at least 10-15 seconds until areddish color appears at the lower edge of the test membrane. NOTE: Ensure the specimens do not pass the maximum line marked on the test strip when immersing the str Please refer to the illustration below (Figure 1).
- Withdraw the test strip and place on a non-absorbent clean and level surface, start the timer and wait for the colored line(s) to appear.
- Interpret the result at 3-8 minutes, do not read the result after 8 minutes.
For Test Card
- Take out the test card from the sealed pouch. Perform the test as soon as soon as possible.
- Remove the cap from the test card.
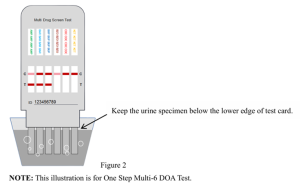
- Hold the test card and immerse the bottom end of the test strip vertically in the specimen for at least 10-15 seconds until areddish color appears at the lower edge of the test membrane. NOTE: Keep the urine specimen below the lower edge of test card when immersing the Please refer to the illustration below (Figure 2).
- Withdraw the test strip from the urine specimen, cover the cap, place on anon-absorbent clean and level surface, start the timer and wait for the colored line(s) to appear.
- Interpret the result at 3-8 minutes, do not read the result after 8 minutes.
For Test Strip assembled in plastic cup
- Open the sealed pouch and take out the plastic Perform the test as soon as possible.
- Remove the lid in anticlockwise direction.
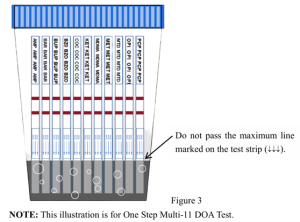
- Identify test strips assembled inside the cup to ensure they are Directly urinate into the test cup, or add the collected urine specimens along the insidewall where there is a scale line marked on the cup. NOTE: Ensure the specimens do not pass the maximum line marked on the test strip. Please refer to the illustration below (Figure 3).
- Cover and tighten the lid on the cup in clockwise direction, place the cup on a level surface, start the timer and wait for the colored line(s) to appear.
- Interpret the result at 3-8 minutes, do not read the result after 8 minutes.
Quality Controls
- An internal quality control is included in the test, in the form of a colored line appearing in the control line region (C), indicating that the testis functional, and proper and sufficient volume of specimen has been applied to enable migration through the test and control lines, regardless of whether there is a test line or not. If the control line (C) does not appear within the testing time, test result is invalid and the test should be repeated with a new test.
- The use of external controls is recommended to verify proper test kit Quality Control samples should be tested with each new lot according to the quality control requirements of the testing facility. It is also recommended to test the products in storage monthly. When testing quality control samples, follow the same testing procedure as for testing urine specimens.
Results
Interpretation of Results
- Negative: Presence of both a reddish purple quality control line (C) and a reddish purple test line (T) on the strip indicate a negative test result, or the drug concentration is below limit of detection.
NOTE: This test is a preliminary screening test. A negative result indicates the drug level is below the detection sensitivity. It is essential to understand that concentrations of the drug below the cut-off value may yield a faint “ghost line” to form in the test line region (T). This “ghost line” should be considered a negative test result.
- Positive: Presence of only a reddish purple control line (C) and no reddish purple line at the test line region (T) on the strip indicates that specific drug has been detected and test result is positive.
NOTE: The presence of the control line (C), no matter how faint, within the designated observation time, indicates a positive result.
- Invalid: There should always be a reddish purple line at the quality control line region (C) regardless of test result. If control line (C) is not presented, the testis considered invalid. Repeat the test using a new test device. Clinical consideration and professional judgment should be applied to any drugs of abuse test result, particularly when results are p Positive results should be confirmed by an alternative method such as GC/MS.
Specific Characteristics
- Accuracy
The accuracy of the VeloTM Multi-Drugs Rapid Test were evaluated in each individual test strip and in comparison to GC/MS method for 130 clinical urine specimens, including 110 positive specimens and 20 negative specimens. Each test was performed by three operators. The results of each test are listed below:
| Specimen | AMP | BAR | BZO | BUP | COC |
| Sensitivity | 98.1% 95% CI (93.6%-99.5%) | 100% 95% CI (96.6%-99.5%) | 97.2% 95% CI (92.2%-99.0%) | 96.3% 95% CI (91.0%-98.5%) | 99% 95% CI (95.0%-99.8%) |
| Specificity | 100% 95% CI (83.8%-100%) | 100% 95% CI (83.8%-100%) | 100% 95% CI (83.8%-100%) | 100% 95% CI (83.8%-100%) | 100% 95% CI (83.8%-100%) |
| Accuracy | 98.4% 95% CI (93.6%-99.5%) | 100% 95% CI (97.1%-100%) | 97.6% 95% CI (93.4%-99.2%) | 96.9% 95% CI (92.3%-98.8%) | 99.2% 95% CI (95.7%-99.8%) |
| Specimen | KET | THC | MDMA | MTD | MET |
| Sensitivity | 98.1% 95% CI (93.6%-99.5%) | 100% 95% CI (96.6%-99.5%) | 97.2% 95% CI (92.2%-99.0%) | 100% 95% CI (96.6%-99.5%) | 99% 95% CI (95.0%-99.8%) |
| Specificity | 100% 95% CI (83.8%-100%) | 100% 95% CI (83.8%-100%) | 100% 95% CI (83.8%-100%) | 100% 95% CI (83.8%-100%) | 100% 95% CI (83.8%-100%) |
| Accuracy | 98.4% 95% CI (93.6%-99.5%) | 100% 95% CI (97.1%-100%) | 97.6% 95% CI (93.4%-99.2%) | 100% 95% CI (97.1%-100%) | 99.2% 95% CI (95.7%-99.8%) |
| Specimen | OPI | PCP | TRA | TCA |
| Sensitivity | 98.1% 95% CI (93.6%-99.5%) | 97.2% 95% CI (92.2%-99.0%) | 96.3% 95% CI (91.0%-98.5%) | 98.1% 95% CI (93.6%-99.5%) |
| Specificity | 100% 95% CI (83.8%-100%) | 100% 95% CI (83.8%-100%) | 100% 95% CI (83.8%-100%) | 100% 95% CI (83.8%-100%) |
| Accuracy | 98.4% 95% CI (93.6%-99.5%) | 97.6% 95% CI (93.4%-99.2%) | 96.9% 95% CI (92.3%-98.8%) | 98.4% 95% CI (93.6%-99.5%) |
- Precision
The precision of VeloTM Multi-Drugs Rapid Test were determined by conducting the test with spiked controls and interpreted the results by three individuals to verify the random error of visual interpretation. The results of 50 specimens each of 50% above and 50% below cut-off specimens are 100% agreed by three observers. The test results were found to have no significant differences between the three observers.
- Analytical Sensitivity
The cut-off concentrations (sensitivity level) of each indicator of the VeloTM Multi-Drugs Rapid Test is determined to be: 1000ng/ml (AMP), 300 ng/ml (BAR), 300 ng/ml (BZO), 10ng/ml (BUP), 300ng/ml (COC), 1000ng/ml (KET), 50ng/ml (THC), 500ng/ml (MDMA), 300 ng/ml (MTD), 1000 ng/ml (MET), 300 ng/ml (OPI), 25 ng/ml (PCP), 200 ng/ml (TRA) and 1000 ng/ml (TCA).
- Analytical Specificity
The specificity study for each of the indicator of the VeloTM Multi-Drugs Rapid Test was evaluated separately by adding structurally related compounds to normal human urine, and all of them produced positive results when tested at levels equal or greater than the concentrations listed below:
| Tests | Compounds | Concentration |
| Amphetamine (AMP) | d-Amphetamine | 1000 ng/ml |
| I-Amphetamine | 25 μg/ml | |
| d,I-Amphetamine | 625 ng/ml | |
| (±)3,4-Methylenedioxyamphetamine | 1 μg/ml | |
| (±)Phenylpropanolamine (PPA) | 4 μg/ml | |
| Phentermine | 1 μg/ml | |
| Barbiturates (BAR) | Secobarbital | 300 ng/ml |
| Alphenol | 150 ng/ml | |
| Aprobarbital | 37.5 ng/ml | |
| Barbital | 300 ng/ml | |
| Butabarbital | 300 ng/ml | |
| Butalbital | 75 ng/ml | |
| Phenobarbital | 300 ng/ml | |
| Phentobarbital | 300 ng/ml | |
| 5,5′-diphenylhydantoin | 300 ng/ml | |
| Benzodiazepines (BZO) | Oxazepam | 300 ng/ml |
| α Hydroxyalprazolam | 300 ng/ml | |
| α Hydroxyaltriazolam | 300 ng/ml | |
| Alprazolam | 100 ng/ml | |
| Bromazepam | 400 ng/ml | |
| Clobazam | 3000 ng/ml | |
| Clonazepam | 1000 ng/ml | |
| Clorazepate | 100 ng/ml | |
| Desmethyldiazepam | 100 ng/ml | |
| Diazepam | 100 ng/ml | |
| Flunitrazepam | 400 ng/ml | |
| Flurazepam | 150 ng/ml | |
| Lorazepam | 300 ng/ml | |
| Lormetazepam | 400 ng/ml | |
| Medazepam | 1500 ng/ml | |
| Nitrazepam | 400 ng/ml | |
| Nordiazepam | 300 ng/ml | |
| Prazepam | 150 ng/ml | |
| Temazepam | 300 ng/ml | |
| Triazolam | 750 ng/ml | |
| Buprenorphine (BUP) | Buprenorphine | 10 ng/ml |
| Buprenorphine 3-D-Glucuronide | 15 ng/ml | |
| Norbuprenorphine | 20 ng/ml | |
| Norbuprenorphine | 200 ng/ml | |
| Cocaine (COC) | Benzoylecgonine | 300 ng/ml |
| Cocaine | 15 μg/ml | |
| Ecgonine | 100 μg/ml | |
| Tropacocaine | 100 μg/ml | |
| Ketamine (KET) | Ketamine | 1000 ng/ml |
| Marijuana (THC) | 11-nor-△-9-THC-9- COOH | 50 ng/ml |
| 11-nor-△-8-THC-9- COOH | 50 ng/ml | |
| △8-THC | 1800 ng/ml | |
| △9-THC | 2000 ng/ml | |
| Cannabinol | 5000 ng/ml | |
| 11-hydroxy-△9-THC | 10 μg/ml | |
| 11-hydroxy-△8-THC | 10 μg/ml | |
| Methylenedioxymethamphetamine (MDMA) | Methylenedioxyampphetamine (MDA) | 2000 ng/ml |
| MethylenedioxyethylMDMA (MDEA) | 1000 ng/ml | |
| L-MDMA | 100 ng/ml | |
| d-MDMA | 100 ng/ml | |
| L-methMDMA | 100 ng/ml | |
| d-methMDMA | 100 ng/ml | |
| HydroxymethMDMA (HAM) | 100 ng/ml | |
| DihydroxymethMDMA (HMMA) | 100 ng/ml | |
| N-methyl-1(1-3-benzodioxol-5-yl)-2-butanamine (MBDB) | 100 ng/ml | |
| Methadone (MTD) | (±) Methadone | 300 ng/ml |
| Methamphetamine (MET) | (+) Methamphetamine | 1000 ng/ml |
| (±) Methamphetamine | 1.0 μg/ml | |
| (±)3,4-Methylenedioxymethamphetamine | 1.0 μg/ml | |
| (±)3,4-Methylenedioxyamphetamine | 10 μg/ml | |
| d-amphetamine | 5 μg/ml | |
| d, I-amphetamine | 10 μg/ml | |
| Ephedrine | 25 μg/ml | |
| Pseudoephedrine | 10 μg/ml | |
| Phenylpropanolamine (PPA) | 50 μg/ml | |
| Opiates (OPI) | Morphine | 300 ng/ml |
| Morphine-3-d-glucuronide | 300 ng/ml | |
| Hydromorphone | 300 ng/ml | |
| Nalorphine | 300 ng/ml | |
| Codeine | 500 ng/ml | |
| Ethylmorphine | 500 ng/ml | |
| Hydrocodone bitartrate | 1000 ng/ml | |
| Norcodeine | 2000 ng/ml | |
| Normorphine | 3700 ng/ml | |
| Oxycodone | 2500 ng/ml | |
| Heroin | 4000 ng/ml | |
| Naloxone | 6000 ng/ml | |
| Thebaine | 5000 ng/ml | |
| Phencyclidine (PCP) | Phencyclidine | 25 ng/ml |
| Naloxone | 20 μg/ml | |
| Tramadol (TRA) | n-Desmethyl-cis-tramadol | 200 ng/ml |
| o-Desmethyl-cis-tramadol | 10,000 ng/ml | |
| Cis-tramadol | 100 ng/ml | |
| Phencyclidine | 100,000 g/ml | |
| Procyclidine | 100,000 ng/ml | |
| d,I-O-DesmethyI venlafaxine | 50,000 ng/ml | |
| Tricyclic Antidepressants (TCA) | Tricyclic Antidepressants (TCA) | 1000 ng/ml |
- Interferences
The following substances were tested and confirmed did not interfere with the VeloTM Multi-Drugs Rapid Test when tested at the listed concentrations.
| Glucose | 2000 mg/dl |
| Bilirubin | 2 mg/dl |
| Human Albumin | 2000 mg/dl |
| Hemoglobin | 10 mg/dl |
| Uric Acid | 10 mg/dl |
| Urea | 4000 mg/dl |
- Cross-reactivity
The following compounds show no cross-reactivity with the VeloTM Multi-Drugs Rapid Test when tested at concentration up to 100 µg/ml (100,000 ng/ml) unless specified.
Acetaminophen, 4-Acetamidophenol, Acetylsalicylic acid, Amikacin, Arterenol, Aspartame, Ascorbic acid, Atrophine, Caffeine, Camphor, Chloroquine, Chlopheniramine, Cortisone, Deoxyephedrine, Dextromethorphan, Digitoxin, Digoxin, Diphenhydramine, Ecgonine, Ecgonine Methyl Ester, Ephedrine, Epinephrine, Gentisic acid, Guaiacol Glyceryl Ether, Histamine, Hydrochlorothiazide, Homatrophine, Ibuprofen, Isoproterenol, Lidocaine, Meperidine, Methaqualon, Methylphenidate, Neomycin, Niacinamide, Perphenazine, Penicillin G, Phenylethylamine, Phenylpropanolamine, Promethazine, Pseudoephedrine, Quinine antidine, Salicyclic acid, Tetracycline, Tetrahydrozoline, Theophyline, Thioridazine, Trifluoperazine, Tryptophan, Tyramine.
Limitation of the Procedure
- The VeloTM Multi-Drugs Rapid Test is designed to be used for the detection of DOA and/or their metabolites with unadulterated human urine specimens only.
- There is a possibility that other substances and/or factors,e.g. technical or procedural errors, may interfere with the test and cause false results.
- Contaminated or tainted urine specimen may give false results.
- The test cannot determine the quantitative drug level or concentration in urine specimen.
- The test provides only a qualitative, preliminary analytical result. A secondary analytical method must be used to obtain a confirmed Gas chromatography/mass spectrometry (GC/MS) is the preferred confirmatory method.
- Adulterants, such as bleach and/or alum, in urine specimens may produce erroneous results regardless of the analytical method If adulteration is suspected, the test should be repeated with a new test device and another new urine specimen.
- Apositive result does not indicate level or intoxication, administration route or concentration in urine.
- A negative result may not necessarily indicate drug-free urine. Negative results can be obtained when drug is present but below the cut-off level of the test.
- The test does not distinguish between drugs of abuse and certain medications.
- A positive result might be obtained from certain foods or food supplements.
Storage and Safety
- Store as packaged at room temperature or refrigerated (2°C-30°C), away from direct sunlight.
- The test is stable through the expiration date printed on the sealed pouch and outer package (24 months from date of manufacture).
- DO NOT FREEZE or expose the kit to temperatures over 30°C.
- Perform the test immediately after taking out the test from the foil pouch or canister.
NOTE: For test strips in canister package, once the canister has been opened, the remaining test(s) are stable for 90 days only.

