Velo™ hCG Pregnancy Rapid Test (Midstream)
Catalog Number: VE242003(2Preps)
Package Specification: 2 tests/kit
Principle
VeloTM hCG Pregnancy Rapid Test adopts the principles of double antibody sandwich method and immunochromatography to test the human chorionic gonadotropin (hCG) in the urine. The membrane is pre-coated with anti-hCG antibodies in the test region (T) and anti-mouse antibodies in the control region (C). During testing, the urine sample reacts with the colored conjugate (mouse anti-hCG antibody colloidal gold conjugate) which has been pre-coated on the test strip. The mixture then migrates upward on the membrane to react with anti-hCG antibodies in the test region and generate a red line. Presence of the red line indicates a positive result, while its absence indicates a negative result. Regardless of the presence of hCG, as the mixture continues to migrate across the control region, a red line at the region will always appear. The presence of this red line serves as verification for sufficient sample volume and proper flow and as a control for the reagents.
Sample Collection and Preparation
The first morning urine is preferred since it generally contains the highest concentration of hCG. However, urine at any time of day may be used.
Procedure
- Read the entire procedure carefully prior to performing any tests.
Allow test midstream to equilibrate to room temperature (4-30℃) prior to testing.
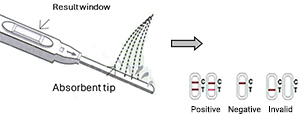
- Remove the test stick from its foil pouch by tearing at the notch and take the cap off the test stick.
- To perform the test, turn the test stick so the absorbent tip is pointing in a downward direction and hold the test stick in your stream of urine, so the urine makes contact with the absorbent area. Hold it for at least ten seconds so that adequate urine goes into the absorbent part of the tip. Do not allow urine to splash into the result window.
- Re-cap the pregnancy test stick and lay the test stick flat on a horizontal surface with the result window facing upwards.
- Wait for red lines to appear. The test should be read in approximately 3-5 minutes. Do not interpret results after 5 minutes.
Quality Control
A procedural control is included in the test. A red line appearing in the control region (C) is considered an internal positive procedural control. A clear background in the results window is considered an internal negative procedural control. It is recommended that a positive hCG control (containing 25-100 mIU/ml hCG) and a negative hCG control (containing “0” mIU/ml hCG) be included in each day testing to verify proper test performance.
Results
- Positive: Two distinct red lines will appear, one in the test region (T) and one in the control region (C). Positive test results are possible but not definitive for the diagnosis of pregnancy. It is strongly recommended that an additional urine specimen be obtained after 48-72 hours and tested again, or consulting your physician.
- Negative: Only a single red line appears in the control region (C). No apparent red or pink line appears in the test region (T). Negative test results mean that you may not be pregnant. However, you should re-test with the first morning specimen obtained 48-72 hours later.
- Invalid: Control band fails to appear which means improper testing procedures or deterioration of reagents probably occurred. In any event, repeat the test. If the problem persists, discontinue using the lot immediately and contact your local distributor.
Specific Characteristics
- Sensitivity: The analytical sensitivity of VeloTM hCG Pregnancy Test is 25mIU/ml and will detect hCG concentrations between 25mIU/ml to 500,000mIU/ml. The sensitivity was established by repetitive testing of samples containing 25mIU/ml hCG during a period of several weeks.
- Specificity: The specificity of VeloTM hCG Pregnancy Test was determined from cross-reactivity studies with known amounts of Luteinizing Hormone (hLH), Follicle Stimulating Hormone (hFSH), and Thyroid Stimulating Hormone (hTSH). Negative results were obtained from all tests conducted with 500 mIU/ml hLH, 1000 mIU/ml hFSH, 1000 mIU/ml hTSH and negative hCG Specimen.
- Precision: A study was conducted which consisted of performing a series of replicate assays using 25 mIU/ml and 100 mIU/ml hCG in urine. All the test results were consistent.
- Diagnostic sensitivity and specificity: Studies were performed which consisted of testing 126 positive and 177 negative specimens using VeloTM hCG Pregnancy Test versus a reference hCG immunoassay. Both of these studies demonstrate 100% (relative) correlation.
Limitation of the Procedure
- False-negative readings can occur when testing is done too early. To get the most accurate results, it is a good idea to wait for about a week after your period is due before testing. This allows more hCG to build up in your urine, which will allow for a more accurate test.
- Drinking too much fluid before taking the test may cause a false-negative result. For the most accurate results, take the test first thing in the morning when your urine is the most concentrated.
- Certain fertility medications which contain hCG (such as Pregnyl, Profasi, Novarel) can give a false-positive result. Alcohol, oral contraceptives, birth control pills, analgesics (pain killers), antibiotics or hormone therapies that do not contain hCG should not affect the test result.
- A number of medical conditions other than pregnancy, including ovarian cyst, choriocarcinoma or ectopic pregnancy (pregnancy outside the uterus) can cause elevated levels of hCG.
- Using the test within 8 weeks of giving birth or having a miscarriage may also cause a positive result. You should ask your doctor for help in interpreting your test result if you have had the experience of pregnancy described above recently.
Storage and Safety
- Store as packaged in the sealed pouched at room temperature (4-30℃). The kit is stable within the expiration date printed on the labeling.
- Once open the pouch, the test midstream should be used within 2 hours. Prolonged exposure to ambient humidity will cause product deterioration.

